Characteristics and Uses of Zirconium Hydroxide
zirconium hydroxide
Molecular formula: Zr(OH)2
Molecular weight: 159.25

Zirconium hydroxide is white and amorphous powder. When heated to 500℃, zirconium oxide (ZrO2 _ 2) is generated after losing two molecules of water. Soluble in dilute inorganic acid, insoluble in water and alkali solution. It can be prepared by the interaction between zirconium nitrate or zirconium sulfate and alkali. Its preparation generally adopts high-temperature furnace drying and calcination, which not only has high investment and energy consumption, but also has serious pollution, easy agglomeration, low purity and uneven particle size distribution.
Solubility: insoluble in water and alkali, soluble in organic acids and inorganic acids, calcined at high temperature to become zirconium dioxide.
Specs
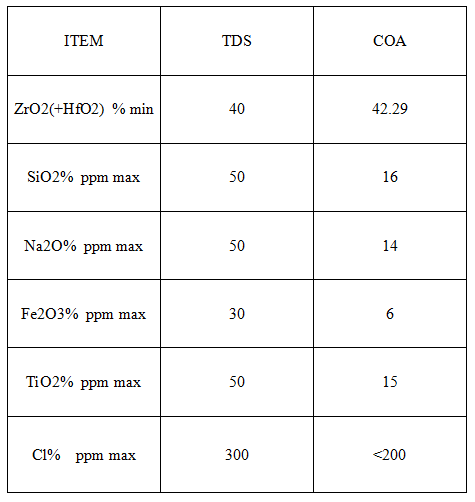
Application: It is mainly used to prepare zirconium and zirconium compounds, and also used as fillers, catalysts, deodorants and pigments in plastics, rubber, ion exchange resins and other industries.Used in the preparation of zirconium compounds, pigments, dyes and glass industries.
